GUY'S AND ST THOMAS'S
MEDICAL AND
DENTAL SCHOOL
Department of
Dental Materials
Science
GUY'S
HOSPITAL
LONDON
BRIDGE
LONDON
SE1 9RT
TELEPHONE:
071 9554549
FAX:
071 357 7563
Head of Departnent
D
Brown MSc PhD CEng MIM
0719554551
M
Sherriff BSc PhD MPRI ANCRT
071
9554550
R
V Curtis BSc PhD DIC
071
955 4548
Professor
N E Waters MSc PhD CPhys Finst p
071
9554548
DBROWN@UK.AC.LON.UMDS.PORTIA
DBrown@uk.ac.lon.umds.falstaff
12th December
1992
Dear Mr
Thank you for
your letter regarding the voltage produced whenever metals are in contact.
Whenever such systems exist an electrical circuit also exists, and for a current to flow this circuit needs to be complete. Even on the microscopic scale such circuits always exist, and what we always need are two electrodes ( an anode and a cathode), an electrolyte (solution of ions) and an electrically conducting connection between the electrodes:
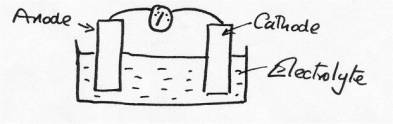
Under these
conditions, provided the anode and the cathode are different metals, a
voltage difference will exist, and when the circuit is complete a current will
flow [as indicated on the meter}. When Galvani did the experiment his ‘meter'
was the leg of the frog, which also acted as a source of electrolyte. The two connected
metals combined to produce both the electrodes and the electrical
connection.
When such a
current flows in a Galvanic Cell the driving force has to come from somewhere,
and the source of this force is the production of electrons at the anode, in
the process we describe as corrosion. Hence:
Metal A - 1 electron = A+ [ an ion of
metal A], or it may be
Metal B - 2 electrons = B++ [ an ion
of metal B]
At
the other electrode (the cathode) another reaction occurs, and this is usually
associated with the decomposition of the electrolyte. For example,
2H+
[ions in water] + 2 electrons = H2 [hydrogen gas]
Only when both
reactions can occur does a current flow, and the reason why batteries [for
example] don't last for ever is that the paste electrolyte becomes saturated
with the products of these galvanic reactions and the electrodes effectively
become poisoned.
If, like me,
you have amalgam fillings, then you are probably well aware of these galvanic
currents when the foil from chocolate gets in contact with them, or we touch
our amalgam fillings with the tang of a fork. My own mouth contains gold crowns
as well as amalgams, but I am only made aware of their potential for creating
galvanic cells when I complete the circuit with a metal artefact. Here at Guy's
[as in all dental schools] we teach our student dentists to avoid placing gold
and amalgam in our patients such that they are in permanent contact in saliva -
one of the body's electrolytes.
Your sincerely,
Dr David Brown
Senior
Lecturer in Dental Materials Science


(This page was http://book.boot.users.btopenworld.com/brown.htm )