The
Setting Reaction
The
setting reaction between the Ag-Sn alloy and the mercury is initiated by a
vigorous mixing of the two ingredients. This mixing causes the outer layer of
the alloy particles to dissolve into the mercury, forming two new phases which
are solid at room temperature. The reaction is as follows:
Ag3Sn
+ Hg ®
Ag3Sn + Ag2Hg3 +
Sn7Hg
g
+ mercury ®
g + g1 +
g2
powder liquid unreacted
amalgam matrix
alloy
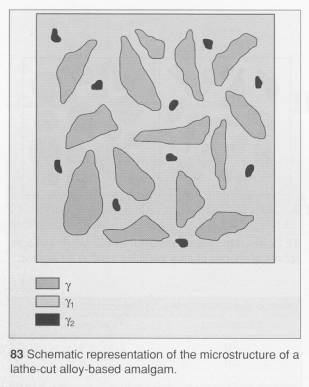
As can be seen from the
reaction, not all of the alloy dissolves in the mercury. On the contrary, a
considerable amount remains, so that the final structure is one of a core of g held together by a
matrix of predominantly g1 which is interspersed
with g2.
The structure of the set material is shown in 83.
The copper in the
lathe-cut alloy is present in the form of discrete areas of Cu3Sn,
and remains mainly within the original alloy in its unreacted form.
In the case of the
spherical particles, the copper is uniformly distributed, and the alloy could
be more accurately regarded as a ternary alloy of silver, tin and copper.
Hence, in the final structure of the spherical alloy amalgam, the copper is not
present as a discrete phase, but is widely distributed throughout the material.
Although some voids will inevitably be present, in a well condensed amalgam
there will be very little porosity.
Introduction
to Dental Materials, Richard van Noort, Mosby, 1994, ISBN 0 7234 1963 9
(This page was http://book.boot.users.btopenworld.com/setting.htm )